2023.12.29
Preventing Rust on Planetary Gearboxes: A Guide for Long-Term Maintenance

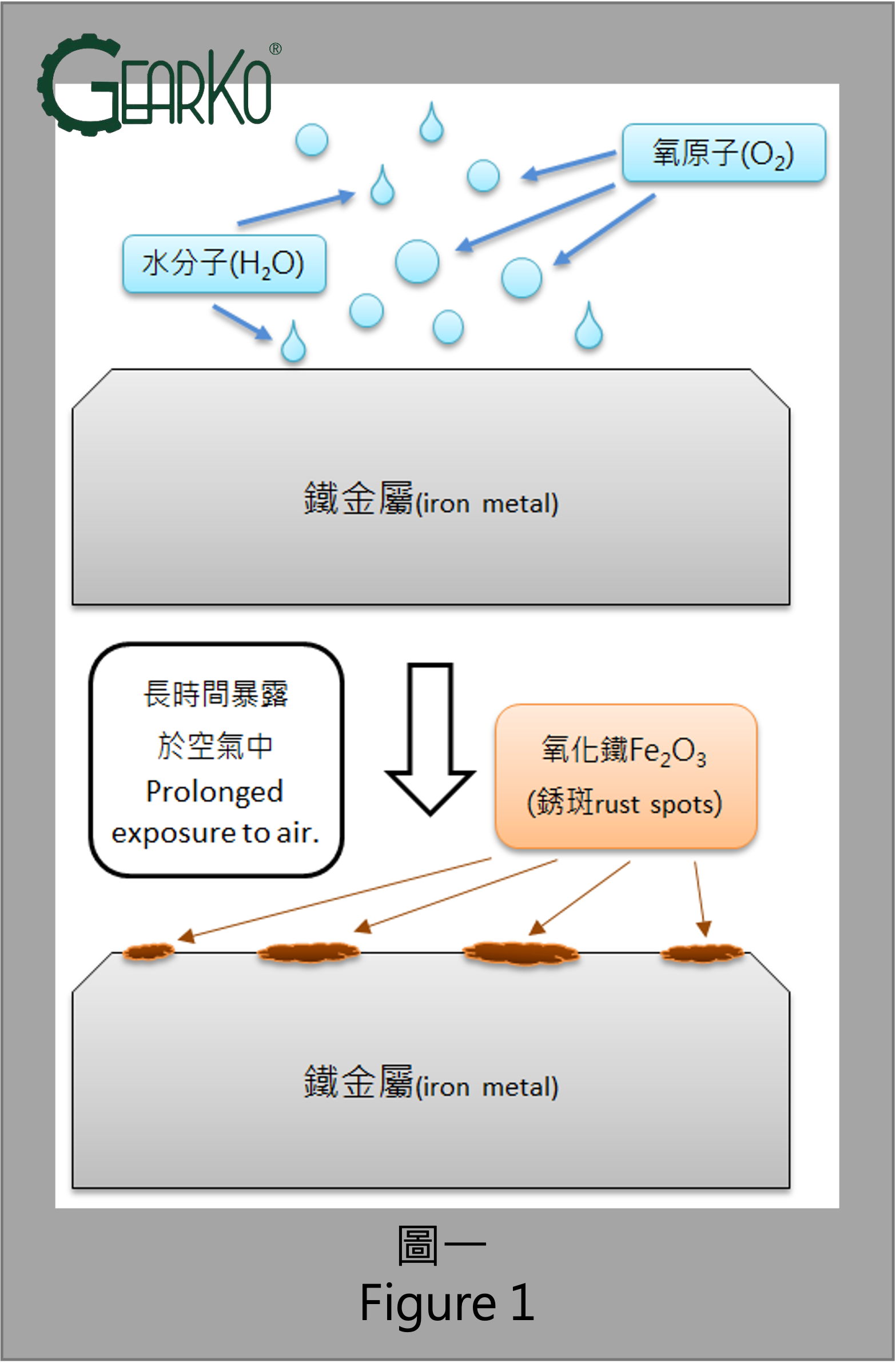
Rust, in essence, is the result of the oxidation process between metal (including carbon steel, stainless steel, aluminum, copper, nickel alloys, etc.) and the air and moisture in the atmosphere. Carbon steel, commonly known as "iron," undergoes oxidation when exposed to oxygen atoms in the air. Due to the easy electron stripping capability of oxygen atoms from iron, a stable state is formed on the surface of iron, resulting in the formation of iron oxide, as depicted in Figure 1. The appearance of iron rust is typically a dark reddish-brown color, as illustrated in Figure 2.

Iron rusts, but what about aluminum and stainless steel? Many believe that aluminum doesn't rust, but, in fact, aluminum is more prone to rusting than iron. However, unlike the visible scars caused by iron rusting, aluminum easily forms aluminum rust, creating a dense layer of aluminum oxide on the surface. This layer adheres tightly to the aluminum, preventing further oxidation and resulting in a phenomenon known as passivation, maintaining a visually appealing appearance.
Stainless steel, defined by its chromium content (requiring at least 10% chromium to be classified as stainless steel), with the addition of other elements to enhance specific mechanical properties, is not entirely immune to rusting. Despite its designation as stainless steel, its corrosion resistance is due to the formation of a thin and robust chromium-rich oxide film on the surface, preventing the infiltration of oxygen atoms and providing good weather resistance and corrosion resistance. Once rust corrosion occurs in stainless steel, it penetrates much deeper than the surface rusting in carbon steel (iron), leading to a more profound corrosion.
Process of Rusting After Painting:
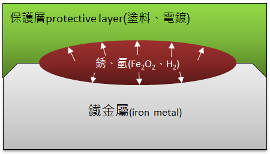
1. Due to the inability to completely remove rust from the metal surface and the entrapment of iron rust within the protective layer, water in the composition dissociates into H+ and OH-. This process continues to produce iron oxide, as shown in the diagram below.

2. After rusting, the volume of iron rust expands by 40-50%, and due to the dissociation process, hydrogen gas is generated, creating internal pressure that accelerates the squeezing of the protective layer.
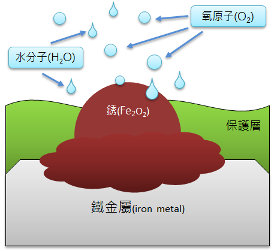
3. Once the protective layer is breached, it comes into contact with air, absorbing moisture and oxygen, accelerating the rusting process and expanding the rusted area.
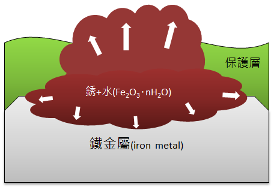
4. Rusting with air produces iron (III) oxide and hydrates, further hastening the destruction of the metal substrate and protective layer until rust removal and repainting occur.
Efforts to Prevent Rust:
In the past, the belief was that isolating air and water from the external environment would prevent rusting. However, metals begin rusting as soon as they are produced, and contact with air initiates rust production. Even with surface treatments such as painting or electroplating, rusting cannot be completely halted. The best we can do is to extend the time it takes for metal surfaces to rust.
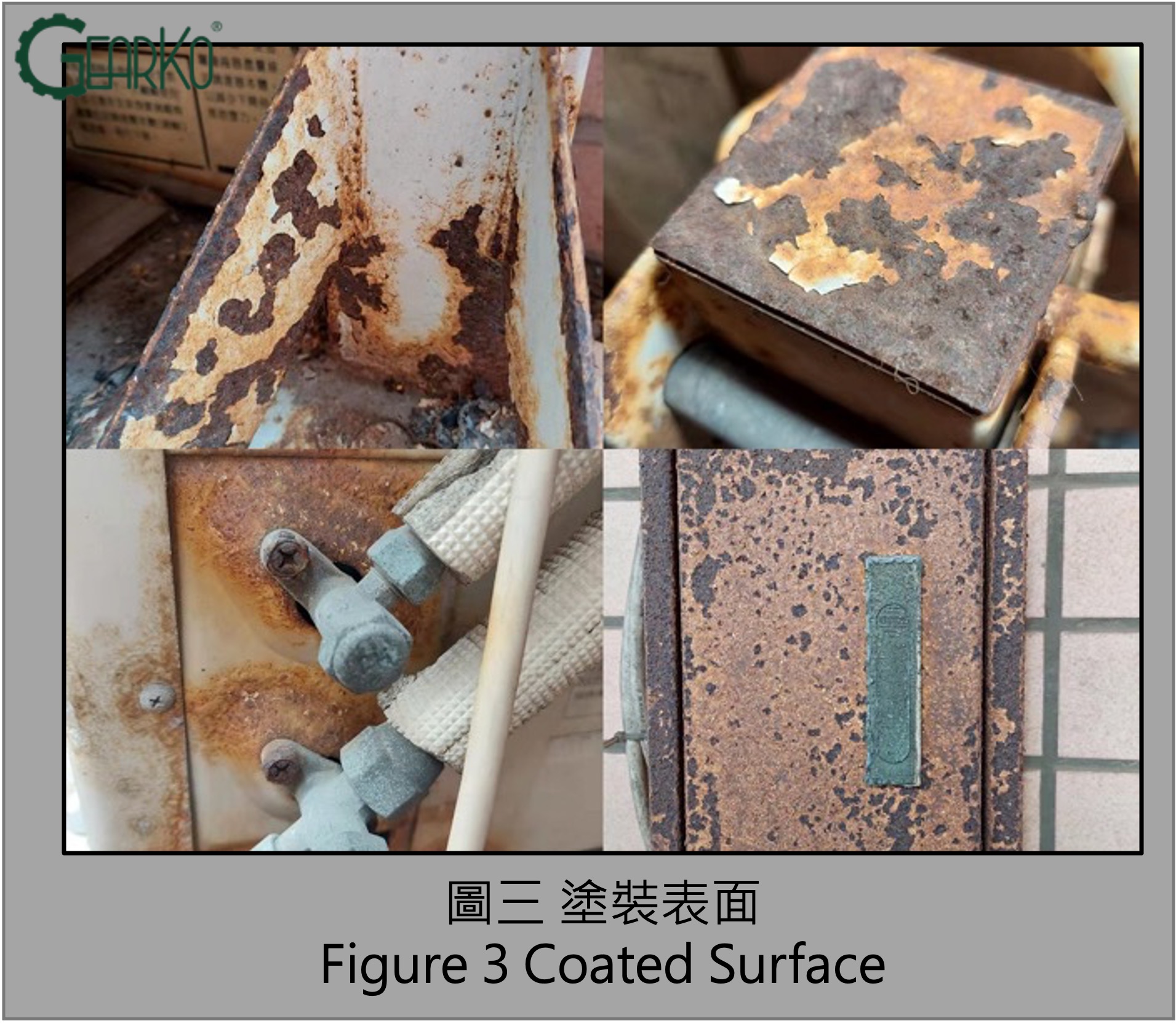
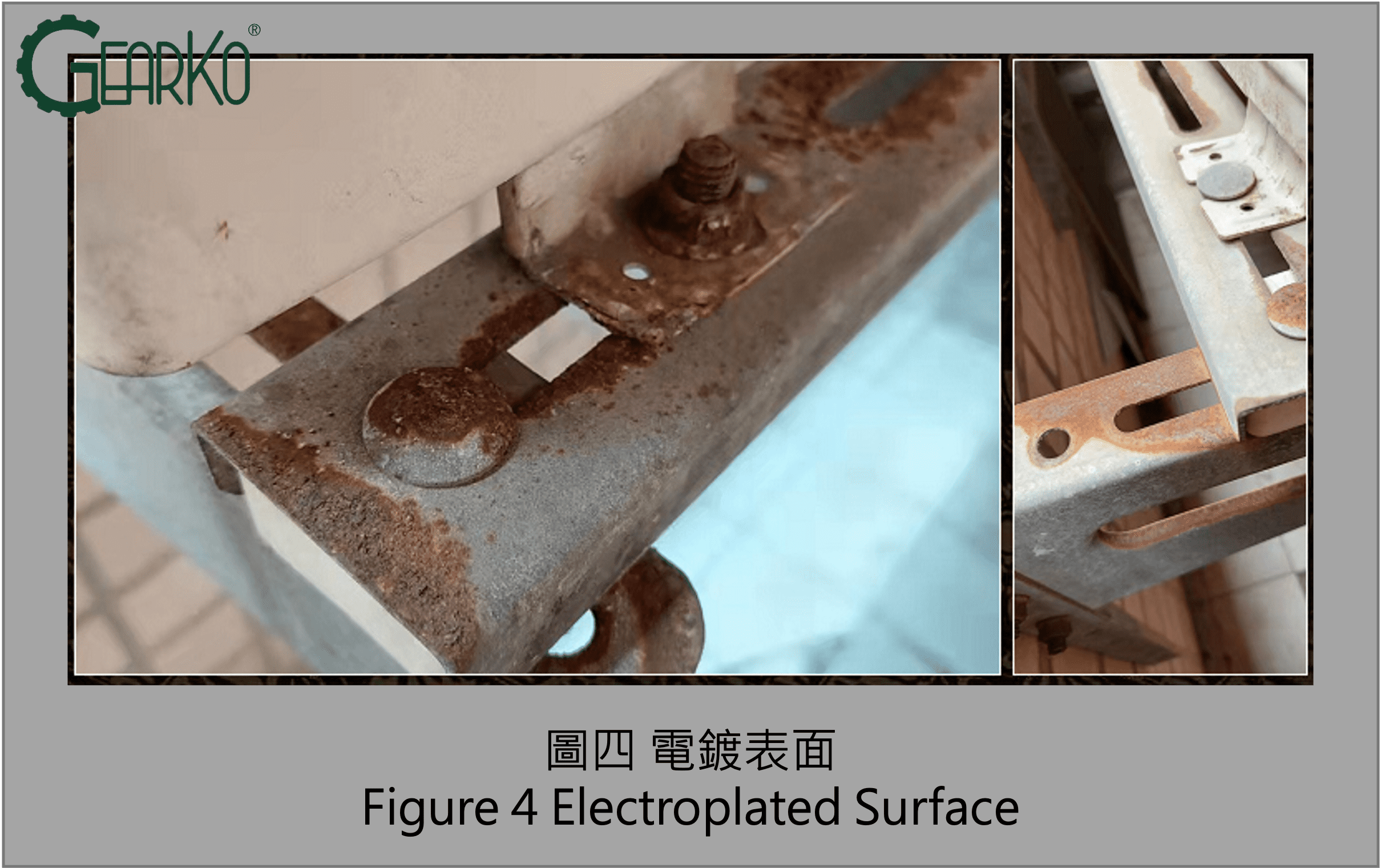
The Most Prone Areas for Rusting in Planetary Gearboxes:
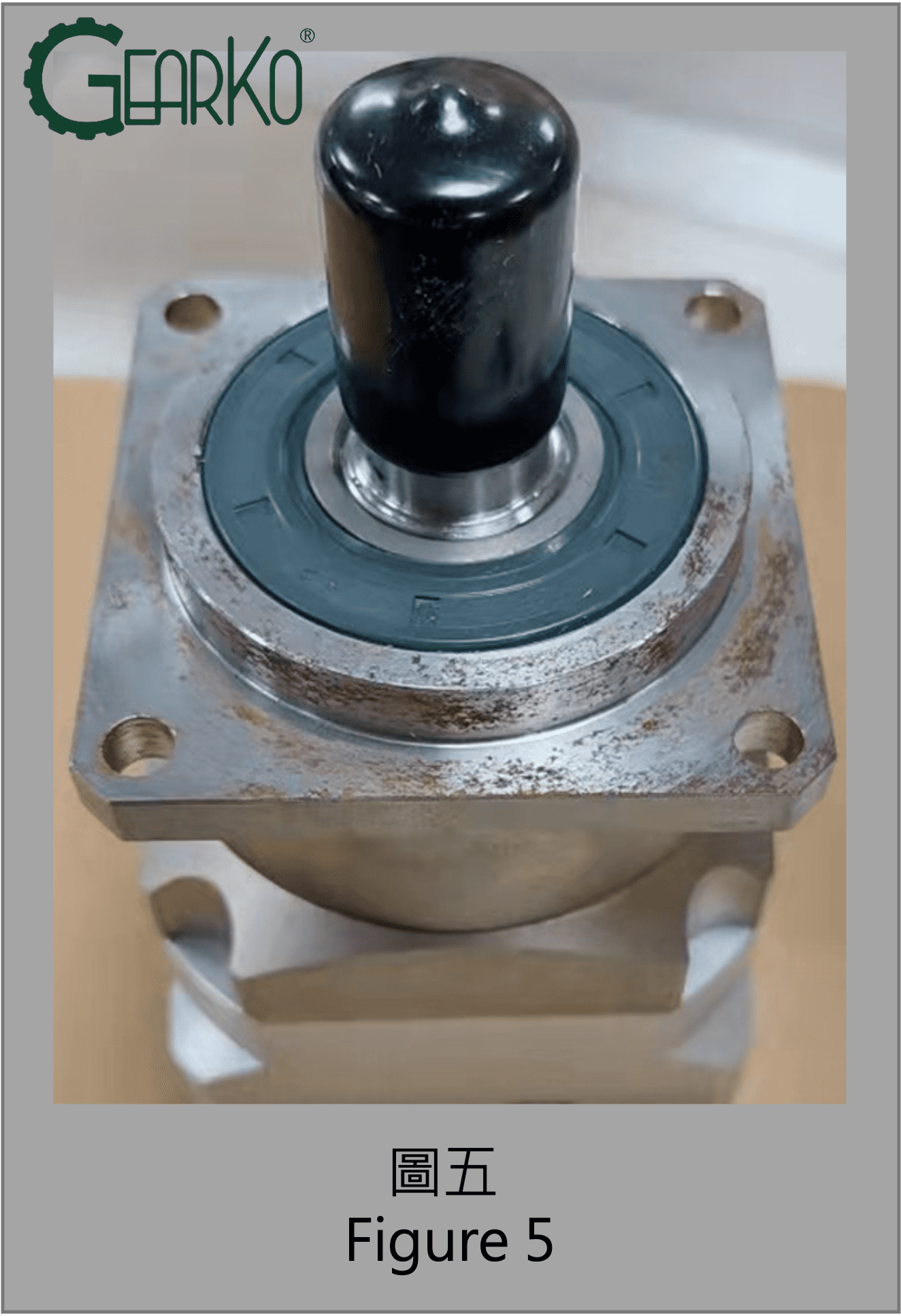
Because planetary gearboxes are precision transmission components, surface treatments are not applied to dimensions requiring high geometric precision to avoid compromising accuracy. Therefore, critical dimensional positions during installation, such as the output flange face and shaft center, as seen in Figures 5 and 6, are the most susceptible to rusting.

Methods to Prevent Rust on Precision Planetary Gearboxes can be summarized into five points:
1. Maintain a dry and well-ventilated environment: Control humidity to limit moisture in equipment or indoor air to prevent condensation. In industries operating in high-temperature and humid conditions, air conditioning and dehumidifiers may be necessary.
2. Keep the gearbox body clean and dry: For planetary gearboxes not in use for extended periods, apply rust preventive oil on metal surfaces to isolate them from the air. Additionally, using plastic wrapping during storage further protects against air contact, ensuring prolonged storage.
3. Regular lubrication: Appropriate lubrication creates a protective oil film on metal surfaces, preventing direct contact with oxygen and moisture, thus preventing rusting.
4. Prevent continuous corrosion: If rust has already occurred, promptly remove the rust and apply suitable rust-preventive coatings to prevent excessive rusting and corrosion of components, enhancing the longevity of planetary gearboxes.
5. Regular maintenance and inspection: Periodically check for rust and corrosion on gearbox components, replacing or repairing damaged and aging parts promptly. Regular maintenance not only extends the lifespan of planetary gearboxes but also enhances their performance and efficiency.
In conclusion, due to the presence of air and moisture in the atmosphere, metals start rusting from the moment they are manufactured. This applies not only to planetary gearboxes but also to all machinery. Preventing rust in mechanical equipment is crucial. In addition to regular maintenance, efforts can be directed toward preventing humidity, corrosion, regular lubrication, and the installation of protective covers. Effective rust prevention ensures stable and prolonged use of mechanical equipment to meet production demands.
For more information on the surface treatment processes of aluminum alloys and metals, refer to the knowledge-sharing articles on Taiwan Gear High's website, specifically "Metal Surface Treatment Topic: The Role of Anodizing in Planetary Gear Reducers" and "Metal Surface Treatment Topic: Environmental Benefits and Advantages of Electroless Nickel" These articles provide detailed explanations.